Sciatica
scRNA-seq generates a molecular map of emerging cell subtypes after sciatic nerve injury in rats | Communications Biology – Nature.com
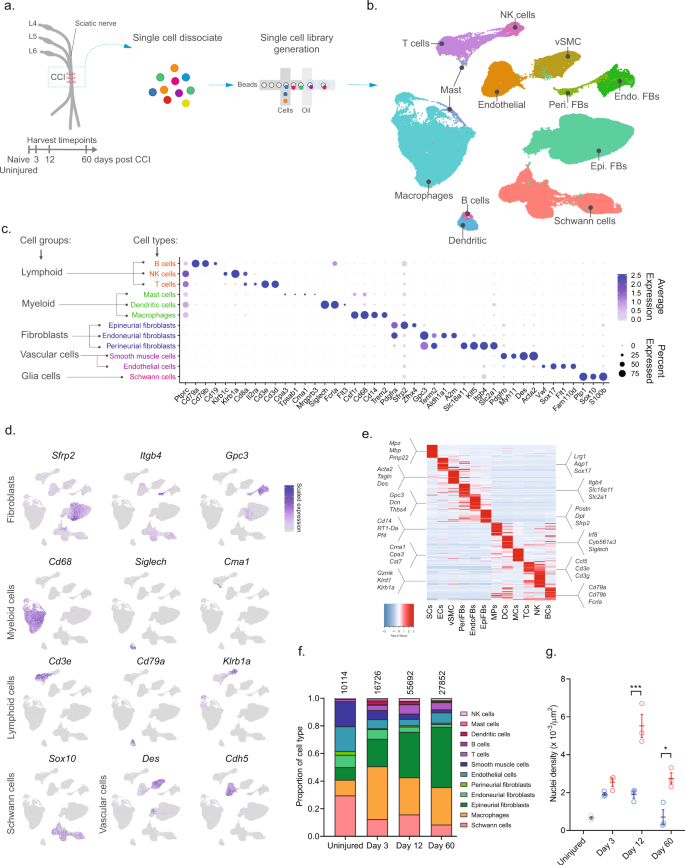
To investigate the cellular subtypes in naive nerve and those that emerge after nerve injury, we used scRNA-seq of enzymatically dissociated naive and injured rat sciatic nerve at three discrete timepoints post chronic constriction injury (CCI; 3, 12, and 60 days post CCI) (Fig. 1a). We chose these timepoints based on the development of mechanical allodynia and thermal hyperalgesia in the CCI model present at day 3, 12, and 60 post-injury10,11,12. At day 3 and 12, early and late phases of acute inflammation, respectively, is also relevant for the development of edema, cell infiltration, and proliferation. At day 60 post-injury, acute inflammation have subsided, some animals may experience a partial recovery of nociceptive hypersensitivity, but scarring of nerve persists. To ensure only tissue-adherent cells were profiled, but not circulating cells, we transcardially perfused the animals before tissue extraction, which resulted in a near elimination of red blood cells indicative of removal of circulating blood cells (Supplementary Fig. 1). From each timepoint, we sampled between 10,351 and 62,101 cells, and between 3 and 7 biological replicates (Supplementary Table 1) totaling 112,521 cluster-assigned cells for the entire dataset. We achieved high-quality data enabling us to identify ~2600 median genes per cluster across the entire dataset (Supplementary Fig. 1). Given the high number of cells and multiple timepoints assayed, our annotation strategy consisted of first using unsupervised clustering of all cells from all timepoints to classify every cell as a member of a major cell group (Fig. 1b), and then subsequently re-cluster individual major cell groups to explore biology within them.
a Experimental outline of dissociation of sciatic nerve from naive animals and from CCI injured animals at 3, 12, and 60 days post-injury. b UMAP plot of clustered cells from all timepoints (n = 112,521 cells, n = 33 10X libraries, n = 18 animals, n = 4 timepoints). c Dot plot of scaled expression of genes differentiating major cell types and classification of their group. d Feature plot of cell type markers. Cells plotted in random order. e Heat map displaying scaled expression of top 20 differentially expressed genes among major cell types using cluster average expression values. f Proportion of cell types in scOmics data at each timepoint. g Nuclei density in nerve tissue at each timepoint quantified by histology (blue = contralateral, red = ipsilateral, gray = uninjured, individual values and mean ± SD, n = 5 technical replicates per animal, n = 3 animals, two-way ANOVA with Sidak’s multiple comparisons test, ***p < 0.0001, *p < 0.0035).
Unsupervised clustering identified 32 clusters (Supplementary Figs. 2 and 3) that based on known markers and differentially expressed genes could be classified into five major cell groups described as lymphoid cells (B cells, NK cells, and T cells), myeloid cells (dendritic cells, mast cells, and monocytes), fibroblasts (epineurial, perineurial, and endoneurial fibroblasts), vascular cells (smooth muscle cells and endothelial cells), and Schwann cells (Fig. 1c–e). Using this approach, we classified >99% of the cells. Each cell type comprised cells from all timepoints, except for a very small group of cells which we termed mast cells based on markers expressed in the uninjured state (Supplementary Fig. 4). The resulting identification of cell types was consistent with previously published descriptions of the cellular anatomy of sciatic nerve, suggesting our protocols recovered cells irrespective of type. We did not recover neurons, for which the cell body reside in the dorsal root ganglion and were not within scope of the current effort and have been published elsewhere13.
In the naive nerve, we identified 36.1% vascular cells, 29.6% Schwann cells, 20.6% fibroblasts, 11.5% myeloid cells, and 2.2% lymphoid cells. While every major cell type was represented at each timepoint, there were significant changes in the proportion among them after injury (Fig. 1f). Importantly, using histology we determined that a significant increase in cell density was elicited by injury at days 12 and 60 (Fig. 1g), which may be explained by infiltration and proliferation of cells and should factor into the interpretation of relative proportion of type in Fig. 1f.
Schwann cells respond to injury by proliferation and differentiation into a transient repair-like phenotype
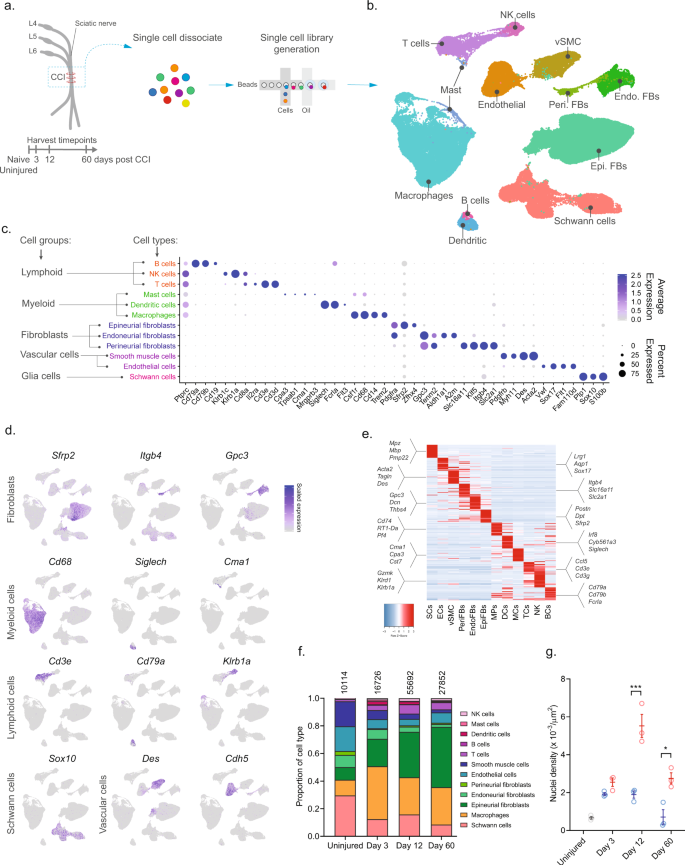
To explore biology within cellular subtypes in each major cell group including cell proliferation and infiltrating types before and after injury, we next subclustered individual cell groups across all timepoints. We first subclustered the Schwann cell group (16,259 cells) and identified 15 clusters all of which expressed pan-Schwann cell markers (S100b and Sox10), except for cluster 9 (746 cells) which comprised putative doublets (Fig. 2a and Supplementary Fig. 5). Based on known markers, we were able to further classify each cluster into one of the following subtypes: myelinating Schwann cells (Prx14), non-myelinating Remak Schwann cells (Gfap15), dividing Schwann cells (Mki6716), repair cells (Ngfr, also known as p75NTR17), as well as a cluster of cells we termed transition cells (Fig. 2b, c and Supplementary Fig. 5). All the Schwann cell subtypes expressed specific gene signatures further supporting they are subtypes, except for the transition type, which likely consisted of multiple transitional phenotypes with a temporal signature (Fig. 2d, e).
a UMAP plot of Schwann cells from all timepoints (n = 16,259 cells). Top: All timepoints. Bottom: Individual timepoints. b Violin plot with boxplot overlay and outliers (dots) of markers identifying Schwann cell subtypes across all timepoints. c Dot plot of scaled expression of markers identifying subtypes. d Heat map of the distribution (percentage) of each subtype normalized to each timepoint. Mean ± SD superimposed on each cell. Left row labels and top column labels indicate cell number in cluster and timepoint, respectively. e Heat map displaying scaled expression of top 20 differentially expressed genes among Schwann cell subtypes across all timepoints. Expression is scaled. f Violin plot with boxplot overlay and outliers (dots) of specific markers identifying myelinating and Remak Schwann cell subtypes among Schwann cells. g Violin plot with boxplot overlay and outliers (dots) of specific markers identifying Repair Schwann cell subtypes among Schwann cells. h ISH of Sox10 RNA on uninjured and injured (3, 12, and 60 days post CCI) nerve. Dashed line indicates the perineurial barrier with the endoneurium above. Injured timepoints show only endoneurium and asterisks indicate ligature-induced necrosis. Scale bar, 100 µm. i Bar chart with dots of Sox10 + nuclei density in uninjured (dark gray), contralateral (light gray), ipsilateral whole area (blue), and ipsilateral proximal to necrotic area (red) (n = 3 animals, mean ± SD, two-way ANOVA with Dunnett’s multiple comparison test, *p < 0.05, **p < 0.01, ****p < 0.0001).
In naive nerve, 94% of Schwann cells classified as either myelinating (58.4%) or non-myelinating Remak Schwann cells (36.1%) (Fig. 2d). We furthered identified 0.8% dividing Schwann cells and 4.7% transitional cells suggesting that ~1% of Schwann cells undergo homeostatic renewal in naive nerve. We also identified several markers that were selective to naive Remak and myelinating Schwann cells, respectively, compared to all Schwann cell subtypes and all major cell types across all timepoints. In myelinating Schwann cells, we identified 14 markers which included Prx and several other markers harboring a more selective profile including Cldn19, Cntf, Drp2, Mrap, Dusp15, and Slc36a2 (Fig. 2f and Supplementary Fig. 6). Surprisingly, while Gfap could be identified as a Remak subtype marker, we failed to identify any markers with properties better than Gfap in terms of higher selective expression compared to other Schwann subtypes and all major cell groups. However, markers that were similar to Gfap included Rxrg, Grin2b, Fxyd7, Ninj2, and Scn7a18 (Fig. 2f and Supplementary Fig. 6).
Interestingly, Schwann cell clusters displayed strong temporal patterning, suggesting that injury triggers molecular changes in Schwann cells. In particular, injury induced the emergence of a transient repair type and a steep increase among dividing Schwann cells (Fig. 2a, d). At day 3 after injury, there was a significant change in the proportion of Schwann cell subtypes including an increase in dividing cells from ~1% to ~20%. This was consistent with histological analysis demonstrating an increase in the density of Sox10 + nuclei within the endoneurium after injury (Fig. 2h, i). The surge of dividing cells coincided with emergence of a neighboring cluster to the dividing one. This injury-specific subtype comprised ~18% of all Schwann cells at day 3 and expressed Ngfr consistent with previously described repair cells19,20. These injury-specific repair cells could be distinguished from others by many differentially expressed genes (Fig. 2e, g and Supplementary Fig. 7). While repair cells were abundant after injury, only one cell among all naive Schwann cells classified as a repair cell, emphasizing that the repair cluster was injury specific. However, the repair subtype was only transient, emerging at 3 days (~18%), persisting at 12 days (~26%), and only sparsely present at 60 days (~3%). Interestingly, dividing cells followed a similar pattern (0%, ~20%, ~6%, and ~2% at naive, 3, 12, and 60 days post injury, respectively). At day 60 post-injury, the proportion of Remak and myelinating Schwann cells had been partially replenished, suggesting that injury triggers cell division and a transient repair cell type in Schwann cells.
We next looked for differentially expressed markers that would selectively label repair cells in injured nerve. Among the 15 most selective genes, we identified several with a known role in neuropathic pain (Cyp2u1 and Lpar321), or in injured and differentiating Schwann cells (Zfp53622, Btc, and Pcsk123), while other selective genes had a more subtle profile associated with nociception (Gpr8324,25, Dusp10, and Tmem196) (Fig. 2g and Supplementary Fig. 7). We also identified a significant increase in genes previously described17,26 in repair cells including Ngfr, Bdnf, Erbb3, and Sox2 (p < 6.4 × 10−214, Fig. 2e) further establishing that these cells are repair cells. Altogether, our data show that in naive injured nerve most Schwann cells comprise Remak or myelinating cells which undergo a low level of renewal during homeostasis. After injury, however, a dramatic increase is evident in cell renewal, which is followed by the emergence of a transient repair cell type at which the nerve undergo elimination of necrotic cells and remodeling.
Anatomically restricted fibroblasts display distinct phenotypical changes after injury
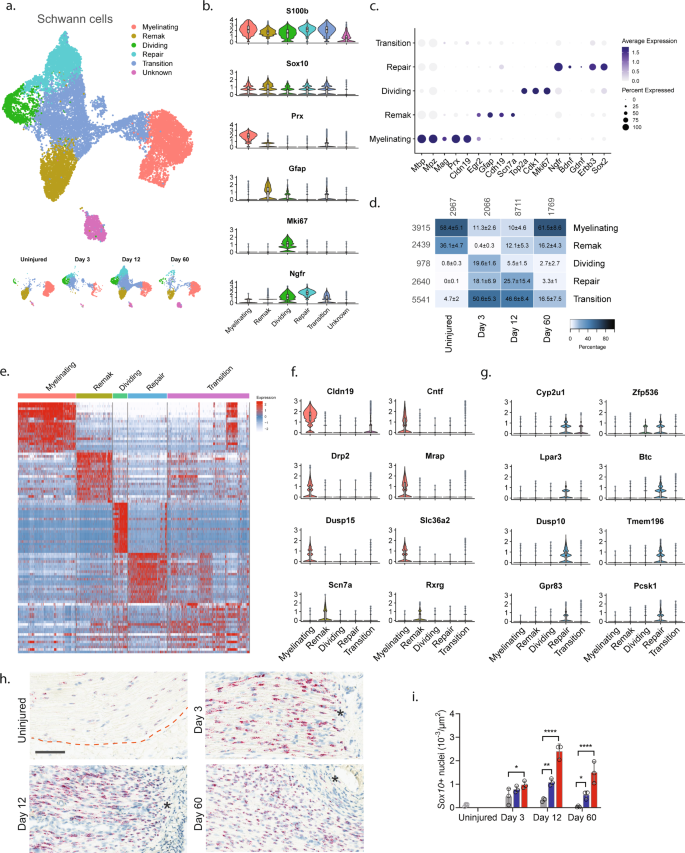
We next subclustered all fibroblasts (40,361 cells) and identified 23 clusters all of which expressed Pdgfra (Fig. 3a, b and Supplementary Fig. 8), except for one cluster which we later identify as injured endoneurial fibroblasts (iEndoFBs type 2577 cells).
a Dimensional reduction plot (UMAP) of subclustered fibroblasts from all timepoints (n = 40,361 cells). Top: All timepoints. Bottom: Individual timepoints. b Heat map of the distribution of fibroblast subtypes at each timepoint (percentage, mean ± SD). Left row labels and top column labels indicate cell number in cluster and timepoint, respectively. c Violin plot with boxplot overlay and outliers (dots) of fibroblast subtype markers. d Feature plot of key markers differentiating epi-, peri- and endoneurial fibroblasts. e Dot plot of scaled expression of fibroblast subtype markers. f, g ISH of Sfrp2 (Scale bar: f, 75 µm; g, 300 µm) and i, j Gpc3 (Scale bar: i, 25 µm; j, 100 µm) in uninjured and injured (3, 12, and 60 days post-CCI) sciatic nerve. Asterisk: Necrotic area. The dashed boxes in (g) and (j) display the image in (f) and (i), respectively. h, k Bar chart with individual values displaying ISH data in (g) and (j) in uninjured (dark gray), contralateral nerve (light gray), ipsilateral – whole area (blue), and ipsilateral – epineurial area (red) for each timepoint (mean ± SD, n = 2–3 animals, two-way ANOVA with Sidak’s multiple comparison test, **p < 0.01, ****p < 0.0001). l, n Heatmaps of top differentially expressed genes in: l the four major fibroblast types, m naive and injured epineurial subtypes, and n naive and injured peri- and endoneurial subtypes. Normalized Z-score.
We first classified each of the 23 clusters into major cell types based on markers associated with the three subanatomical compartments within nerve: epineurial (Sfrp2), perineurial (Gpc3, Itgb4, and Slc2a1), and endoneurial (Gpc3, Sox9, and Aldh1a1) fibroblasts in agreement with previous studies6,27 (Fig. 3c–e and Supplementary Fig. 8). Further, a subset of the epineurial fibroblasts were labeled as dividing (Sfrp2 and Mki6716). We confirmed the spatial patterning of these three major fibroblast types in nerve by histology which demonstrated that Sfrp2 exclusively labeled cells in the epineurium, and Gpc3 exclusively labeled cells in the endoneurium and perineurium, respectively (Fig. 3f–k). These three anatomical types were clearly distinguished by dimensional reduction, regardless of injury state, and displayed differential gene expression when compared to one another (Fig. 3a, l).
In uninjured naive nerve, ~93% of fibroblasts classified as either perineurial (~41%), endoneurial (~13%), or epineurial (~36%) fibroblasts. The remaining cells included dividing and transitional epineurial types. Perineurial fibroblasts establish a physical barrier shielding the endoneurial compartment from the environment outside the nerve. Supporting a role in this function, we identified that perineurial cells expressed several metabolic transporters including glucose (Slc2a1) and monocarboxylate (Slc16a11) transporters (Fig. 3e, l). Endoneurial fibroblasts on the other hand expressed collagens supporting extracellular matrix maintenance (Col9a2, Col11a1, Col15a1, and Col18a1), receptors for CGRP signaling (Calcrl, Ramp1, Ramp3), and Sox9 suggesting a role in stemness28.
In contrast, to the limited types of peri- and endoneurial fibroblasts, epineurial fibroblasts displayed much broader diversity. To this end, we identified six subtypes of epineurial fibroblasts in naive nerve based on dimensional reduction and differential gene expression (Fig. 3a, m).
Interestingly, nEpiFB (naive epineurial fibroblast) type 1 was enriched for genes (Dpp4, Ptges (PGE2), and Car829,30,31) with a role in inflammation, while nEpiFB type 2 was enriched for triggers of pain and neutrophil recruitment (Ptgs2 (Cox-2), Cxcl132, and Irf133) (Fig. 3m). Subtype nEpiFB type 3 expressed many genes involved in development (Tspan11, Spry134, Cldn135), while type 4 expressed genes with a role in differentiation of progenitors (Gdf1036, Bmp437). Type 5 expressed genes with known function in regulating mesenchymal mobilization and angiogenesis (Bmper38 and Fgf1039,40), as well as Neurotrophin signaling in regeneration (Ntrk241, Ntf342), positioning these cells with a role in axonal regeneration and nerve repair. The last type, type 6, expressed genes involved in extracellular matrix formation including fibulin, elastin, collagen, and osteoglycin (Fbln243, Eln44, Col8a1, Ogn8). Injury triggered a sharp change in the proportion of cells across the different subtypes compared to naive which in part was due to cell proliferation or infiltration (Fig. 3b). At day 3, the relative proportion of dividing cells among all fibroblast types had increased from 0.5% to ~10%. This coincided and preceded a significant increase in the density of Sfrp2 + cells of ~3, ~21, and ~4 fold at day 3, 12, and 60 post-CCI, respectively, when examined by histology (Fig. 3h). Similarly, the density of Gpc3 + cells increased ~2, ~6, and ~3 fold at day 3, 12, and 60 post-CCI, respectively (Fig. 3k). Intriguingly, the proportion of endoneurial fibroblasts decreased from ~40% to only ~2% at day 3. This coincided with the emergence of two previously sparsely populated iEndo types. Type 1, which increased from ~1% in naive to 18%, 5%, and 2% at day 3, 12, and 60 post-CCI, respectively, expressed genes involved in differentiation and wound repair (Sox8, Bmp7, Igsf345, Fgf2r39, Ptprn) (Fig. 3n). iEndo type 2, which changed from ~1% to 5%, 1%, and 0.5% at day 3, 12, and 60 post-CCI, respectively, display differential expression of tens of ribosomal proteins.
Distinct epineurial types also emerged after injury with some differences in the temporal signature when they emerged (Fig. 3b). iEpiFB type 1 cells emerged robustly from ~0.5% in naive nerve to ~24%, ~9%, and ~4% at day 3, 12, and 60 post-CCI, respectively. This cluster was the only neighbor to dEpiFBs in the dimensional reduction plot suggesting that this transient type were newly formed cells. iEpiFB type 1 cells expressed genes involved in fibrosis (Acta2, Des, Tnc46), neuromodulator signaling (Npy1r47, Tac348), and neutrophil chemokine signaling (Cxcl6) (Fig. 3m). iEpiFBs type 2 and type 3 had some overlap of genes involved in B cell, neutrophil, and monocyte chemotaxis as well as hematopoietic stem cells migration (Pdgfra, Tnfsf13b49, Cxcl1250, Ccl751). iEpiFBs type 4 and type 5 appeared to share some expression with nEpiFBs type 6 in addition to also expressing genes involved in remodeling (Mmp11, Mmp14, Col8a1, Col11a1, Cilp52, Cilp2).
By subclustering ~40,000 nerve fibroblasts, we have identified nine subtypes of naive fibroblasts among three subanatomical regions within nerve. After injury we further identified the emergence of seven different types of fibroblasts based on genes with roles supporting immune infiltration, activation, and modulation as well as tissue remodeling. Altogether, our findings highlight the complexity of fibroblast heterogeneity, and shed light into their contribution to homeostasis and repair after nerve injury.
Injury triggers myeloid cell differentiation and infiltration, and the generation of a myeloid scar
We next subclustered all myeloid cells (32,265 cells) which identified 24 clusters all of which expressed the pan-myeloid marker Cd68, except for cluster 19 (386 cells) (Fig. 4a, b and Supplementary Fig. 9). Based on known markers, we were able to classify clusters in naive nerve into the following subtypes: conventional dendritic cells type 1 (cDC1s, Xcr153, and Clec9a54,55), mast cells (Cpa356 and Cma157), patrolling monocytes (Nr4a158,59), dividing macrophages (Mki6716), and macrophages (nMPs1-4, Trem2) (Fig. 4b–e). Macrophages could be subdivided further into either complement expressing (Cd16360, C1q61) or MHC class II expressing (RT162 genes). The complement expressing cells could be divided into two clusters based on differential expression of Cd163, Ccl7, Ccl24, Ccl2, Cxcl1, and Cxcl2 (nMP1, naive macrophages type 1), or Ccl4, Anxa3, Ifngr1, Cxcl16, and Igfbp3 (nMP2) suggesting their involvement in neutrophil and monocyte recruitment. A third cluster of naive macrophages (nMP3) expressed both complement and MHC II molecules, and could not be distinguished based on additional selective molecules, suggesting their phenotype was less specialized. A fourth cluster (nMP4) could be identified by expression of MHC II genes (RT1-Db1, RT1-Bb, RT1-Ba63), consistent with previous studies7. nMP4 also expressed several pro-inflammatory mediators (Ccl17) and enzymes and receptors for leukotriene B4 signaling (Ltb4r and Lta4h) suggesting their involvement in neutrophil and leukocyte recruitment.
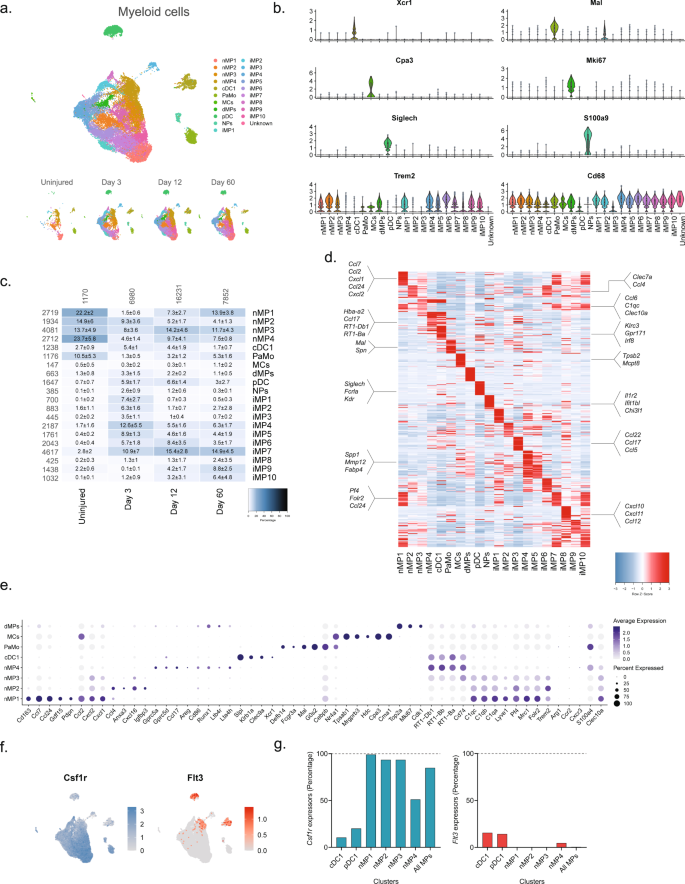
a Dimensional reduction plot (UMAP) of subclustered myeloid cells (n = 32,265 cells). Top: All timepoints. Bottom: Individual timepoints. b Violin plot with boxplot overlay and outliers (dots) of cell type markers. c Heat map of the distribution of each myeloid subtype at each timepoint (percentage, mean ± SD). Left row labels and top column labels indicate cell number in cluster and timepoint, respectively. d Heat map displaying scaled expression of the top 40 differentially expressed genes across cell types from all timepoints. e Dot plot of scaled expression of genes differentiating clusters in naive and injured dominant subtypes. f Feature plot of Csf1rand Flt3 expression distribution across cells from all timepoints, respectively. g Quantification of the percentage of cells expressing (Csf1r, left) and (Flt3, right) in relevant clusters.
Interestingly, the naive macrophage clusters displayed a distinct pattern of Clec10a and S100a4, markers which in another study labeled epineurial macrophages and recently recruited monocyte-derived macrophages, respectively9, suggesting that nMP1-3 are epineurial and nMP4 are recruited. Intriguingly, the nMP4 cluster also displayed expression of two different myeloid lineage markers, Flt3 and Csfr1, indicating that unlike other nMP types (or injury types) which did not express Flt3, the nMP4 phenotype is populated by macrophages deriving from both embryonic precursors and bone marrow lineages (Fig. 4f). In dendritic cells (cDC1 and pDC1), which derive from the hematopoietic lineage, Flt3 could be detected in about 15% of cells suggesting this gene could be underestimating the percentage of hematopoietic derived cells (Fig. 4g). While this result suggests that most nerve macrophages (~85%) derive from early development in line with previous studies9, it also shows that both lineages can differentiate into one phenotype as determined by dimensionality reduction. Thus, here we describe four transcriptionally distinct subdivisions of tissue-resident naive macrophages, adding two previously uncharacterized types, and further identify evidence suggesting that the MHC II macrophage phenotype can be populated by different lineages.
Injury triggered the emergence of several distinct myeloid subtypes driven by phenotypical changes or division of resident cells, and infiltration of additional cell types (Fig. 4b). Histological analysis revealed a dramatic increase in the density of Cd68 + cells after injury which subsided toward naive levels at 60 days, except for highly necrotic areas which remained encapsulated by Cd68+ cells (Fig. 5a, b). At three days after injury several clusters emerged including plasmacytoid dendritic cells (pDCs, Siglech64) and neutrophils (S100a865, S100a965,66) as expected at this timepoint. To verify that this was not just a confound of dissociation technique, we inspected the density of Siglech + cells in nerve. Our analysis revealed the emergence of pDCs cells which peaked at day 12 post injury, consistent with observations in the single-cell data (Contra- vs. ipsilateral at days 3, 12 and 60, respectively: 0.08 ± 0.04 vs. 0.33 ± 0.16, 0.05 ± 0.03 vs. 1.32 ± 0.80, and 0.005 ± 0.001 vs. 0.14 ± 0.05 cells (10−3/µm2), n = 2–3 animals) (Fig. 5c, d). The emergence of neutrophils and pDC1s was accompanied by numerous injury-specific clusters of macrophages some of which appeared transiently while other were more persistent over the time course investigated (Fig. 4c). These clusters were distinguishable based on markers and differentially expressed genes (Fig. 4d).
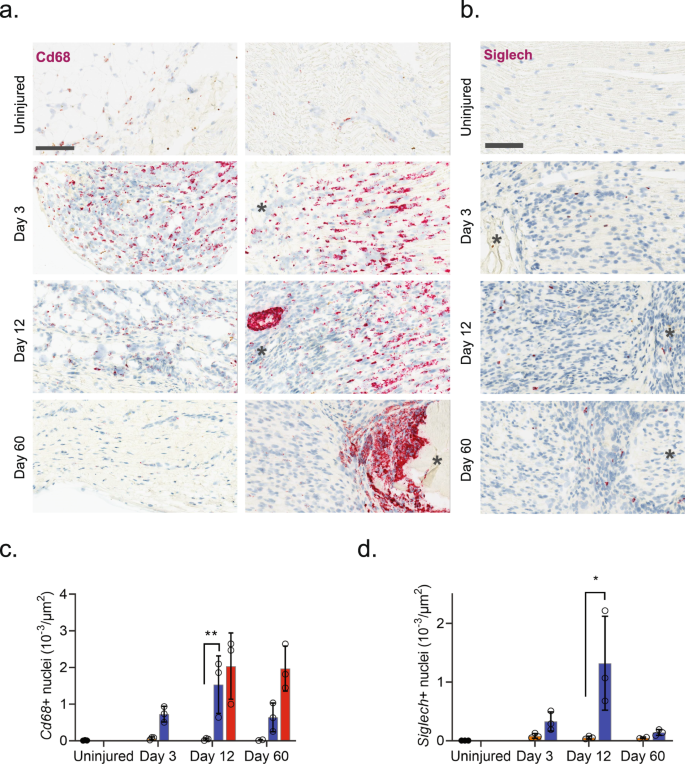
a ISH of Cd68 in uninjured and injured (3, 12, and 60 days post-CCI) sciatic nerve. Column: left, epineurium; right, endoneurium. Asterisk: Necrotic area. Scale bar, 100 µm. b ISH of Siglech in endoneurium of uninjured and injured (3, 12, and 60 days post-CCI) sciatic nerve. Asterisk: Necrotic area. Note that at day 3 nerve does not exhibit necrosis. Scale bars: 100 µm. c, d Bar chart with individual values of ISH data in (a) and (b) in uninjured (dark gray), contralateral nerve (light gray), ipsilateral—whole area (blue), and ipsilateral—necrotic area (red) for each timepoint (mean ± SD, n = 3 animals, two-way ANOVA with Sidak’s multiple test comparison: *p < 0.05, **p < 0.01).
The emergence of injury-specific myeloid subtypes is consistent with the temporal mechanics of inflammatory initiation and resolution in other tissues67. Consistent with these studies we identify the emergence of several transient iMPs (injury-enriched macrophages) and neutrophils at the early timepoint. Injury caused the emergence of neutrophils which could be detected at 3 days after injury. These cells expressed many molecules consistent with a pro-inflammatory profile, including Il1b, Ccl3, and Cxcr2 expression as well as enzymes and receptors for leukotriene B4 signaling (Ltb4r and Lta4h) consistent with autocrine signaling32,67,68,69,70 (Fig. 4d).
However, surprisingly we did not identify a clearly defined cluster with high expression of Ccr2 (Fig. 4e), a factor necessary for monocyte extravasation and inflammatory resolution in other tissues, aside from very scarce expression in iMP2s71. Previous studies in rodent nerve have shown that Wallerian degeneration can progress independently of Ccr2 suggesting that nerve might be a specialized tissue where Ccr2 monocyte extravasation does not play a crucial role in inflammation, that Ccr2 is expressed in neurons not captured by our study, or we missed the temporal window for identifying these cells in our studies72.
The appearance of later arriving iMP types is also consistent with the phenotypical adoption of tissue repair programs in resident MPs. In other tissues these include an early appearance of a pro-inflammatory iMP which help control neutrophil life span followed by a phagocytotic iMP type which help remove debris and apoptotic neutrophils. Histological inspection of Cd68 + cells revealed a dramatic increase in myeloid cells after injury and a distinct and temporally dependent distribution of Cd68+ cells within the tissue. This was evident by widespread homogenous distribution of Cd68+ cells within nerve at 3 days after injury. However, at 60 days after injury CD68 + cells displayed a distinct spatial pattern restricted to enveloping necrotic areas (Fig. 5a).
Vascular cells display change after injury with minimal injury enriched subtypes emerging
We next subclustered vascular cells (13,290 cells) into 17 clusters, which all strongly expressed markers73 for vascular smooth muscle cells (vSMC: Acta2, but no Cspg4), pericytes (Acta2 and Cspg4), or endothelial cells (EC: Tie1), except for cluster 14 which expressed Acta2 and Tie1 weakly, but in addition also expressed markers for epineurial fibroblasts (Sfrp2, Pdgfra, Zfhx4), prompting us to annotate this cluster as doublets (Fig. 6a–d and Supplementary Fig. 10). EC marker analysis further identified clear clusters for dividing endothelial cells (Tie1 and Mki6716) as well as lymphatic endothelial cells (LEC: Pdpn74, Lyve, Ccl2175, Prox176). The expression of Ccl21 in LECs is consistent with their role in shuttling neutrophils, dendritic, and T cells to the tissues, and play a role in inflammation. The remaining ECs could be divided into three clusters distinguished by markers (Fig. 6e, f) with nEC1 being the most abundant EC cluster. While we did not investigate the subanatomical relationship of these three clusters, we did, however, observe interesting markers suggesting functional roles that could impact drug uptake: nEC2 expressed Abcb1a, an ATP-dependent efflux pump with broad substrate specificity also known as Pgp suggesting that capillary vessels in either the epi- or endoneurial compartment may decrease drug penetration similar to the blood–brain barrier in the CNS. nEC3 expressed several markers (Gpb2, Gbp5, Ifit2) suggesting a role for this subtype in response to interferon-gamma stimulation77. In contrast to other major cell groups, injury did not seem to trigger the emergence of distinct cell states as evaluated by dimensional reduction. Instead, only one type of ECs (iECs) and one type of vSMC (iSMC) emerged after injury (Fig. 6c). The majority of top DEGs in iECs were mostly ribosomal protein genes (e.g., Rpl34 and Rps15a) and iSMC expressed a less specific profile (Fig. 6f). This prompted us to investigate whether changes occurred within clusters and across timepoints. For this analysis, we only included nECs (nEC1-3), nSMC, and PC all of which had at least 150 cells represented at each timepoint (Fig. 6k), and we identified that these cell types demonstrated increased number of DEGs at Day 3 or Day 12 compared to the uninjured (Fig. 6l). Injury also triggered a 5–6 fold increase in the proportion of both dividing ECs (dECs: Tie1 and Mki67) and SMC (dSMC: Acta2, Cspg4, Mki67) (Fig. 6c), which is supported by an increase in the density of both ECs and SMCs when examined by histology (Fig. 6g, j). In summary, vascular cells displayed the least diversity after injury responding with robust proliferation rather than the emergence of injury-specific subtypes.
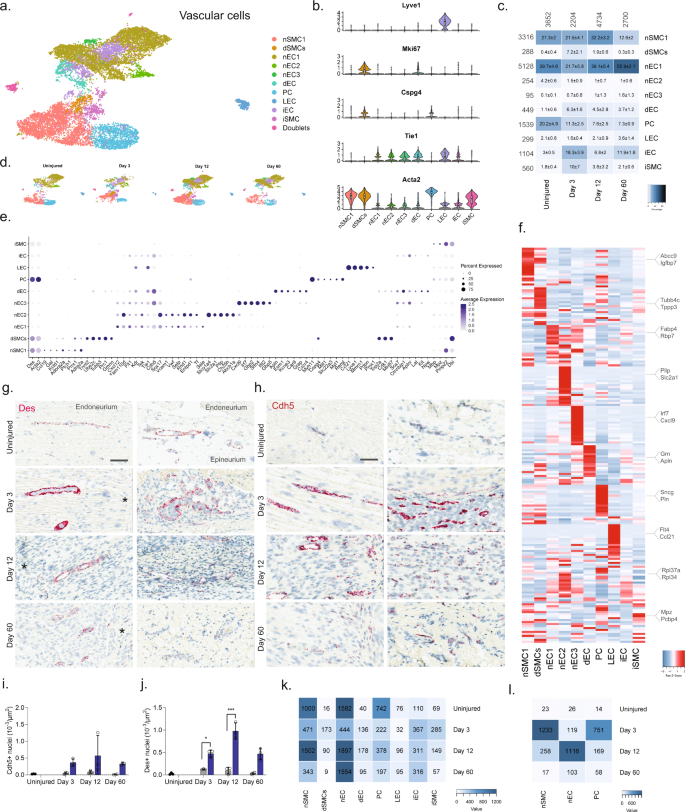
a Dimensional reduction plot (UMAP) of subclustered vascular cells from all timepoints (n = 13,290 cells). Top: All timepoints. Bottom: Individual timepoints. b Violin plot with boxplot overlay and outliers (dots) of cell type markers. c Heat map of the distribution of each vascular subtype at each timepoint (percentage, mean ± SD). d Feature plots of vascular subtype markers. e Dot plot of scaled expression of genes differentiating clusters in among naive and injured subtypes. f Heat map displaying scaled expression of the top 20 differentially expressed genes across cell types from all timepoints displayed from cluster averages. g ISH of Des in endoneurium (left column) and epineurium (right) of uninjured and injured (3, 12, and 60 days post-CCI) sciatic nerve. Asterisk: Necrotic area. Scale bars: 100 µm. h ISH of Cdh5 in endoneurium (left column) and epineurium (right) of uninjured and injured (3, 12, and 60 days post-CCI) sciatic nerve. Asterisk: Necrotic area. Scale bars: 100 µm. i, j Quantification of ISH data in (g) and (h), respectively, displayed as bar charts with individual values: uninjured (dark gray), contralateral nerve (light gray), and ipsilateral—whole area (blue) for each timepoint (mean ± SD, n = 2–3 animals, two-way ANOVA with Sidak’s multiple comparison, *p < 0.05, ***p < 0.001). k Heat map of cell count in each cluster divided by timepoint. l Heat map of absolute count of DEGs for each cell subtype when compared among timepoints. Only cell subtypes with more than 150 cells in each group were included in the analysis.
Unlike T cells, NK cells infiltrate primarily the epineurial nerve compartment
We finally subclustered lymphoid cells which resulted in 17 clusters (Fig. 7a, b and Supplementary Fig. 11). Previous studies of blood white cells have demonstrated the vast diversity and complex expression patterns that is present among this major cell group. In fact, several scOmics studies have demonstrated that combining transcriptomics and proteomics help annotate deeper subtypes among lymphoid subtypes78. In our study, we also observed widespread cluster heterogeneity and decided to not fully characterize clusters beyond major cell types. To this end, we identified eight different subtypes of T cells (Cd3e), five subtypes of NK/ILC1 cells (Ncr1, Klrb1a), ILC2 cells (Pparg), B cells (Cd19), and mast cells (Cpa3) (Fig. 7c–e)55. Interestingly, histological evaluation of the presence of Cd3e + T cells and Ncr1 + NK cells revealed that in uninjured tissue these cells types were essentially absent (Fig. 7f–j), but that injury triggered NK cell infiltration primarily of the epineurial compartment, while T cells could be identified in both the epi- and endoneurial compartments.
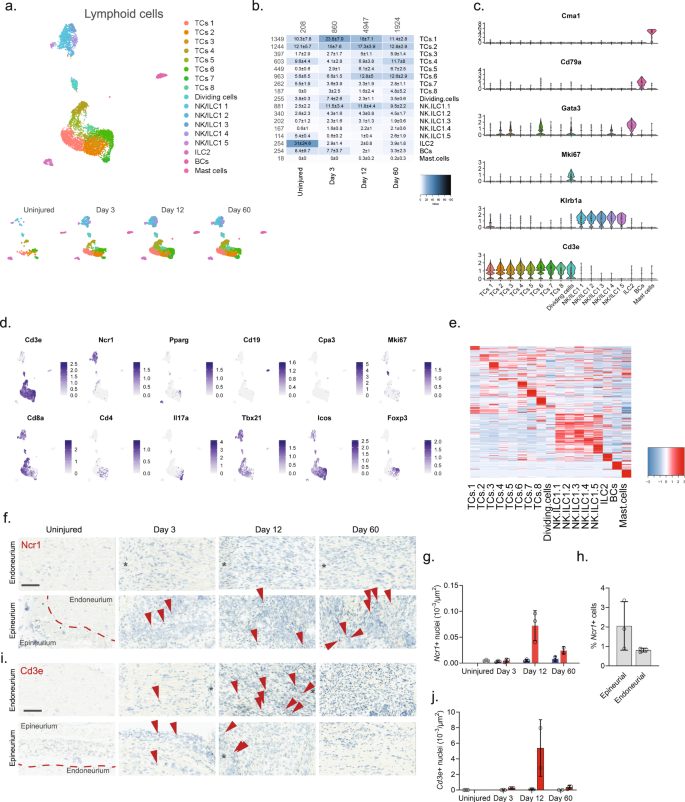
a Dimensional reduction plot (UMAP) of subclustered lymphoid cells from all timepoints (n = 7957 cells). Top: All timepoints. Bottom: Individual timepoints. b Heat map of the distribution of each lymphoid subtype at each timepoint (percentage, mean ± SD). Left row labels and top column labels indicate cell number in cluster and timepoint, respectively. c Violin plot with boxplot overlay and outliers (dots) of cell type markers. d Feature plots of lymphoid subtype markers. e Heat map displaying scaled expression of the top 20 differentially expressed genes across cell types from all timepoints displayed from cluster averages. f ISH of Ncr1 in endoneurium and epineurium areas of uninjured and injured (3, 12, and 60 days post-CCI) sciatic nerve. Asterisk: Necrotic area. Scale bars: 100 µm. g Bar chart with individual values displaying number of Ncr1 + cells in uninjured (light gray), contralateral (blue), and ipsilateral (red, whole area) nerve for each timepoint (mean ± SD, n = 2–3 animals). h Percent Ncr1 + cells in ISH data in (f) in epineurial and endoneurial areas. i ISH of Cd3e in endoneurium and epineurium areas of uninjured and injured (3, 12, and 60 days post-CCI) sciatic nerve. Asterisk: Necrotic area. Scale bars: 100 µm. j Bar chart with individual values displaying number of Cd3e + cells in uninjured (light gray), contralateral (blue), and ipsilateral (red, whole area) nerve for each timepoint (mean ± SD, n = 2–3 animals).
Potential paracrine interactions in anatomically restricted regions increase after injury
We next sought to explore the potential ligand–receptor (LR) interactions that exist between different cell types within naive nerve. We used a bioinformatic framework, SingleCellSignalR, to extract annotated LR pairs derived from a set of curated databases. Using this framework an LR score can be calculated and significant interactions can be identified based on scores between 0.5 and 179. In naive nerve, we observed thousands of potential pairs among all cell types. Interestingly, the majority of LR pairs were between Schwann cells, fibroblasts, myeloid and vascular cells, whereas lymphoid cells occupied a much smaller fraction of potential pairs (Fig. 8a). Given no inflammatory response is anticipated in naive tissues, this observation further establishes confidence that the LR method provides physiologically relevant sensitivity. For exploring interactions between cell types, we focused on Schwann cell and perineurial fibroblasts which displayed anatomically proximity based on our histology data. We identified 112 potential interactions (LR score >0.5) from Schwann cells to perineurial fibroblasts. Among the top pairs (Fig. 8b), we identified the Cntf-Cnftr pair (LR score = 0.84) between myelinating Schwann cells and perineurial fibroblasts (Fig. 8c). Cntf-Cntfr has a known role in nerve development and inflammatory nociception, however, a direct interaction between myelinating Schwann cells and perineurial fibroblasts has not previously been demonstrated (Fig. 8d).
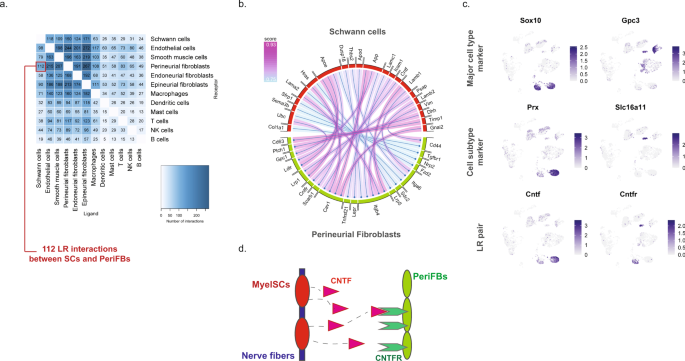
a Number of LR pairs between indicated cell types identified using SingleCellSignalR in naive nerve (LR > 0.05). b Circosplot of top interactions signaling from naive Schwann cells (ligand) to naive perineurial fibroblasts (receptor). c Feature plots validating cell type markers and identified LR pair in all naive cells. d Illustration of signaling between the LR pair, Cntf–Cntfr, between myelinating Schwann cells and perineurial fibroblasts. The LR score between myelinating Schwann cells and perineurial fibroblasts for Cntf and Cntfr is 0.84.
When we explored the number of significant LR pairs after injury, we observed that the number increased especially at day 12 after injury. The distribution also changed with more interactions involving lymphoid and mast cells supporting the activation of an immune response (Fig. 9a–c). Exploring the top most-variable interactions at day 12 and focusing on genes that we identified in injury-specific clusters, we found that the activation of Ngfr in repair Schwann cells might derive from Ngf in endoneurial fibroblasts (Fig. 9d, f). In the same gene set, we identified another interaction between Ptprz1 in repair cells and the ligand Ptn in almost all types of fibroblasts. We also identified a Timp3-Kdr (VEGFR) interaction between injured fibroblasts and endothelial cells suggesting an importance of fibroblasts in the negative regulation of angiogenesis after injury80. Among these LR pairs may be opportunities for pharmacological intervention for alleviating pain after nerve trauma.
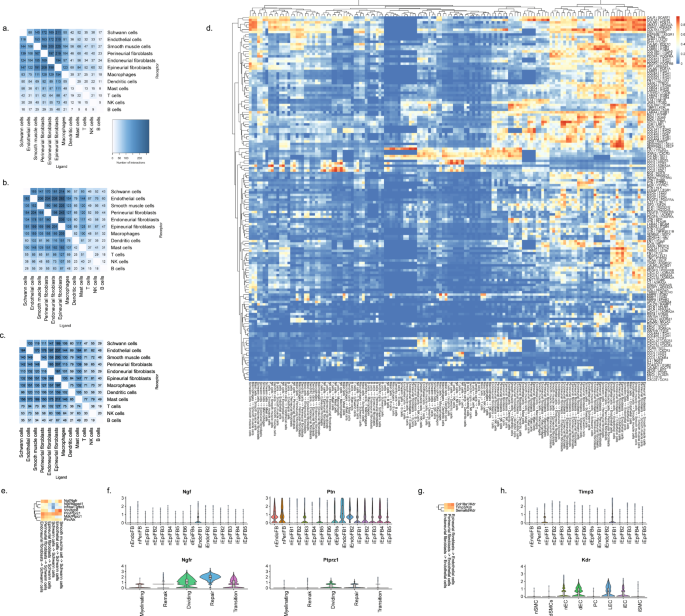
Number of LR interactions larger than 0.5 between the indicated two cell types after a 3 days, b 12 days, and c 60 days of CCI injury, respectively. d Heat map of scaled expression of top 100 most-variable interactions at day 12. e Subset of interactions from (d) between types of fibroblasts and Schwann cells. f Violin plot with boxplot overlay and outliers (dots) of highlighted pairs, Ngf – Ngfr and Ptn – Ptprz1, from (e). g Subset of interactions from (d) between types of fibroblasts and vascular cells. h Violin plot with boxplot overlay and outliers (dots) of highlighted pair, Timp3 – Kdr, from (g).