Around 1percent of the world’s population suffers from celiac illness an allergic reaction to gluten that is caused by a predisposition to hereditary. It is characterised by the presence of intraepithelial lymphocytic infiltration villous atrophy and hyperplasia of the crypt. A variety of clinical manifestations are observed, ranging including gastrointestinal (GI) and neurological signs [11. The most commonly reported GI manifestations include abdominal constipation, abdominal pain, bloating diarrhea, failure to thrive/weight loss, nausea vomiting, and reflux. Extraintestinal manifestations include abnormal liver enzymes, arthralgia/arthritis, dermatitis herpetiformis, alopecia, fatigue, anemia, stomatitis, myalgia, psychiatric disorders, rashes, short stature, delayed puberty, osteoporosis, and infertility [2]. Celiac disease manifests in neurological ways in various ways, such as epilepsy, ataxias, mood disorder, encephalitis neuromuscular disorders, peripheral neuropathy and dementia, as well as learning disorders and delays in development [33. Of all the manifestations migraine is among the primary symptoms of celiac disease.
As per the International Classification of Headache Disorders migraine is a debilitating disorder that can cause mild to severe headaches that last from four to 72 hours, and is often caused by vomiting or nausea. About 17 percent of women suffer from it with migraine, and between 5% and 8 percent of males suffer from it [55. Additionally, it’s associated with a variety of GI illnesses, indicating an enticing link with gastrointestinal and neurological symptoms. This implies that migraines might be related to issues in the gut-brain axis6.
While the precise pathophysiology behind migraine isn’t fully understood the serum of people who suffer from migraine contain an increased amount of pro-inflammatory cytokines like tumor necrosis factors alpha (TNF-a) as well as interleukin (IL)-1 [11. Calcitonin gene-related protein (CGRP) is a peptide that has been shown to be released during migraine attacks, can be located in more than half the the trigeminal ganglion. This is the area where these cytokines exert their influence. Trigeminovascular activity that is repeatedly activated and constant can cause sensitivity to the brainstem nuclei, resulting in the central region being sensitized. In essence it is thought that the pathophysiology behind migraine is caused by series of neurological inflammation, trigeminovascular inflow and central cortico-trigeminal activation [55. In celiac disease the immune system reacts in a way that is hypersensitive to gluten, which results in an autoimmune enteropathy caused by T-cells which causes damage to the small intestine [77. The inflammatory response triggers the activation of enteric neuron and subsequent release of cytokines. One hypothesis is that, instead of being directly mediated by antibodies symptoms of neurological disorders in celiac disease could be caused through a systemic inflammatory response. Therefore, the increased levels of interferon-gamma as well as TNF-a that regulate the production of CGRP can be the cause of the connection between celiac and migraine [5The research suggests that the link between migraine and celiac disease may be mediated by.
Neuropeptides are also thought to have antimicrobial effects on a wide range of gut bacteria (including Escherichia coli, Enterococcus faecalis as well as lactobacillus acidophilus) [33. In addition whether gluten is present or not can alter the variety and amount of microbial species in the gut microbiota which could lead to dysbiosis. Through affecting the gut-brain connection and the gut microbiota could play a role in the development of neurological disorders, such as migraine [1010. This article analyzes the effects of celiac disease and changes to the microbiota in the gut on the development of migraine.
Search strategy
We conducted a title search within PubMed, PubMed Central, and Medline with the following keywords such as migraine and celiac disease microbiota, migraine and celiac disease, gluten and microbiota, celiac illness and microbiota, as well as the gut-brain axis. The articles we considered were chosen without any restriction of the date of publication or the type of study, i.e., traditional reviews, systematic reviews studies that are clinical, such as case-control studies or cohort study. The age and ethnicity of the participants were not considered in the research. There were no limitations regarding the search, in relation to demographics. All of the articles picked were written in English. English language.
In this debate we will go over the current knowledge regarding the homeostasis and functional composition in the microbiota that makes up our gut the intestinal microbiota and celiac disease, as well as the interaction between the GI system and Central Nervous System (CNS) and an in-depth description of the gut-related pathways involved in the formation of migraines.
Gut microbiota
“Microbiome” is the term used to describe whole genome of the human microbiota. The diversity of the ecosystem can be maintained thanks to this abounding microbiota community and is thought to be comprised of 100 trillion microorganisms. Around 10 times the number of microorganisms than human cells exist in the adult body and more than the majority of them within the stomach. More than 5000 microbes and over 1000 kinds of microflora have been identified to be part of the human microbiome [13-14]. Bacteria, specifically anaerobic bacteria, rule this ecosystem. However, protozoa, viruses archaea and fungus are also found [15,16and 16. It is defined primarily with two different bacterial types: Bacteroidetes and Firmicutes, but with some minor contribution by Proteobacteria, Actinomyces, Fusobacterium and Verrucomicrobia [1717.
The gut microbiota has multiple reasons. It first is the barrier that protects the intestinal tract that promotes the intestinal microbiota’s health, encourages intestinal epithelial cells’ renewal produce mucus, and also nourishes mucosa by producing short-chain fats (SCFAs) [1819. It also plays a role in building the immune system by activating the inborn immune system at an young age, which leads to the maturation of the gut-associated lymphoid tissue as well as the activation of adaptive immunity through the stimulation of local and immune system-wide responses [19and 19. Additionally, it enhances the synthesis and metabolism of specific hormones, nutrients and vitamins, and plays an important function in poison and drug elimination. A low level of physiological inflammation, and an effective defense against microorganisms is the result of gut microbiota continuing to enhance the immune system in conditions of physiological functioning [2020. Additionally bacteria have a protective role in the gut through the production of Cytokines that may hinder the growth of microorganisms and provide nutrition to pathogens to survive [2121.
Human development and a variety of factors affect how the gut flora alters. Mothers feed their babies with the microbiota of the beginning [2222. The infant’s gut is a diverse flora that is similar to adulthood at the age of one [23,2424]. The composition of the gut microbiota isn’t constant and changes as we get older. While the changes in dynamic vary widely between individuals, macro equilibrium is a common result [2525. Although certain factors, such as an autoimmune illness, infection or illness, drugs and even food, can affect our microbiomes, the beneficial bacterial changes are able to significantly impact the health of an individual [26,2727.
Gluten, gut microbiota, and gut microbiota
In Western countries the world, gluten is an essential food ingredient. Prolamins, which includes glutenin and gliadin is a complex mix of proteins which make up gluten [99. The consumption of gluten has increased significantly over the past century and, with it the incidence of celiac disease substantially increased. If gluten isn’t completely removed, immunogenic peptides get produced and interact with intestinal mucosa and epithelium [28The mucosa and intestinal epithelium are involved. Humans, as a whole, have microbiota of the duodenal and the metabolism of gluten are tightly connected [2929. Certain microorganisms could be the cause of some of the proteolytic activity, based on particular patterns in gliadin breakdown observed in duodenal biopsies taken from celiac patients that have been identified [3030. While the small intestine is home to less diversity of microbes than the large intestine, the luminescent concentration of gluten proteins promotes the proliferation of bacteria that are proteolytic [31].
Due to the large consumption of gluten in Western countries The small intestine is home to numerous gluten peptides. These peptides are food sources for various duodenal bacteria, which can cause dysbiosis in those suffering from celiac diseases [30]. The diversity of bacteria species, diversity and their proportions are altered during celiac disease because of the activation of an immunological pathway that triggers the inflammation [32,3333, 34].
The gut-brain Axis
The bidirectional relationship with the GI systems and CNS is reflected in the phrase ‘gut-brain axis.’ The brain regulates the GI tract’s actions and movements. The effects of hormonal factors on gut function are controlled by the hypothalamus pituitary (HPA) the axis that regulates stress-related responses. It is also believed to be the case that GI system may affect the CNS. The gut system is involved in various brain functions that include the ability to think, behave as well as the perception of nociception. A variety of neurological diseases, including migraines and migraines, have been linked to the malfunction of the gut-brain-axis [34,35].
Migraine and gut microbiota
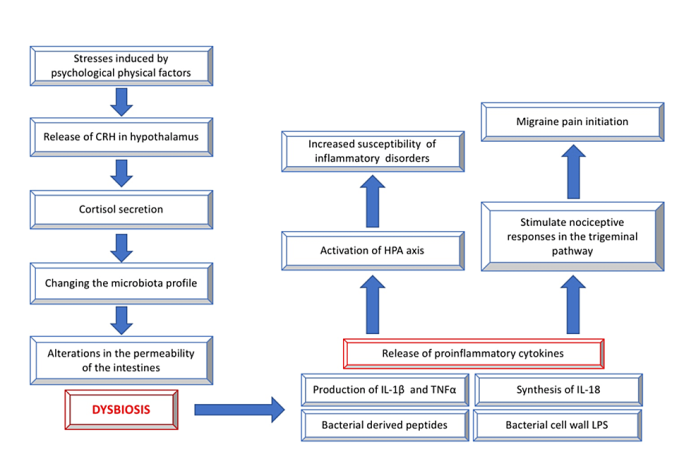
Migraines are one of the neurological diseases that gut microbiome may influence through the gut-brain communications. According to certain studies that gut microbiota could influence the brain-gut axis via different routes, which could affect the behavior of nociceptive neurons and brain function [3433. A columnar intestinal epithelial cells divide the 100 trillion of bacteria on the gut’s surface and the human. The major pathophysiological mechanisms that are linked with migraine are thought to be influenced by the composition of gut microbiota and also play an important part in the gut-brain axis [35]. The hormones, neurotransmitters and inflammatory chemicals that originate from the microbiome are all identified as possible pathways. The figure below illustrates the indirect connections as well as the importance of dysbiosis.
Neurotransmitters
Serotonin, gamma-aminobutyric acid and CGRP are all involved within this process (35-37The process is a result of gamma-aminobutyric acid. Gut bacteria are able to create these neurotransmitters and these substances are exchanged between different cell kinds [38and 39. Particularly, the intestinal cells found in the gut produce large quantities 5hydroxytryptamine (5-HT) which can affect the nervous system [39-4040]. In turn, the microbiome of the human gut produces a variety of neurotransmitters essential to affect the body, specifically in the axis between the brain and gut [4143.
Table 1 summarizes a few studies which have shown the connection between neurotransmitters, gut dysbiosis and neurotransmitters.
Hormones
Through the parasympathetic and sympathetic nervous systems and through the production of neuroendocrine peptides. The CNS can affect the gut flora. Stress-related stress-related issues on physical and mental level can change the microbial composition within the gut. These stressors cause the hypothalamus to release a corticotrophin-releasing hormone, which triggers the adrenal glands to release cortisol. Furthermore, these stressors can alter the microbiota profile and alter the permeability of the intestines, resulting in dysbiosis [51,5252, 53.
The Inflammatory Molecules
Immune cells as well as their inflammatory mediators like IL-1, I-6 and IL-18, TNF a along with interferon-gamma (IFN-g) are found to be able to irritate afferent nerves and can trigger abdominal pain that is visceral [5353. Pro-inflammatory cytokines, such as IL-1 as well as IL-6, I-8 and TNF-a are also linked to migraine and have been found to be elevated in migraine episodes [5453. Gut permeability and inflammation are linked in a bidirectional way which means that increased gut permeability may trigger immune and inflammatory responses via the lipopolysaccharide (LPS) leakage and pro-inflammatory cytokines cause increased gut permeability [55]. Gluten can also affect the nociceptive response in the trigeminal pathway , and may cause the onset of migraine. These cytokines that promote inflammation include TNF-a, IL-1 and the IL-6 [56-6060-60.
Limitations
But, the study does have certain limitations and there’s a lot of questions to be answered. The search strategy used in this review was restricted only to 3 databases i.e., PubMed, PubMed Central (PMC), and Medline. It is theoretically unclear what the gut microbiota’s role is in having any effect on brain functioning. Dysbiosis is apparent in celiac disease, however it’s still not certain if it is the consequence or a cause of it, or if celiac disease exhibits distinctive patterns of changes. This is why more studies are needed to identify the exact effects of dysbiosis , and to determine the pathways that are that are involved in celiac disease-related migraines and migraines, which makes this an intriguing subject to study further.
The connection between celiac disease and migraine is profoundly affected by the gut-brain-axis. The main reason for the prominent involvement of a variety of vasoactive and vasoactive mediators within migraine’s physiopathology is the control of the GI autonomic and immune systems by GI microbiota. Although the mechanism behind this relationship is not fully understood, research suggests that the gut-brain-axis might influence migraines. In general, a variety of elements, including the composition of the microbiota in the gut as well as dietary constituents and neurotransmitters, hormonal pathways and inflammation mediators (IL-1, IL-6, IL-8 and TNF-a) are involved in this interaction. Since certain strains of gut bacteria are recognized to be negatively affected by neuropeptides, such as CGRP It has been speculated that these neuropeptides are associated with the mutual interaction between the brain and the gut. While our understanding has advanced tremendously but it’s still not clear what the effects of higher exposure to gluten and immune system changes and changes in the gut microbiota might affect migraine headaches for people suffering from celiac disease.

We understand how important it is to choose a chiropractor that is right for you. It is our belief that educating our patients is a very important part of the success we see in our offices.