Introduction The condition known as diabetes mellitus (DM) is a major health problem that is common throughout the modern world.1 It has been established by epidemiological...

– Designer Women Global Sciatica Treatment Market trend analysis and precise estimates is included with MarketQuest.biz reports, which provide thorough research alternatives for corporate strategies to...

Dr. Hung K. Lee is seen in the clinic he runs in Solvang. By Dr. Hyun K. Lee Contributing Writer Sciatica is a real discomfort in...


The Village Chiropractic A couple who have previous experience in medical offices decided to start a chiropractic practice of their own after the launch on April...


Chiropractors are health professionals who use spinal manipulation and other techniques to help their patients achieve and maintain good health. They can help people with a...


The back injury suffered by Chicago Cubs top prospect Brennen Davis is more serious than originally believed. Davis was undergoing back surgery on Thursday at Los...


Charlotte – This week only I’ve had two patients visit me to seek the relief they need from the sciatic nerve pain. The majority of people...

The Daily Advance
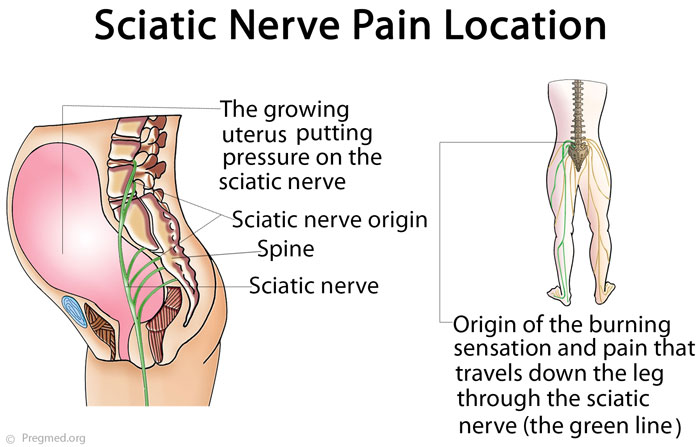

Do You Get Sciatica When Pregnant? Sciatic sensations may increase during your pregnancy. In fact, lower back pain and sciatic problems are quite common. Sciatica will...


Charlotte – In the last week, I’ve seen two clients visit me to seek some relief from their Sciatic nerve pain. The majority of people consider...